RAL-Life is a fast-growing integrated pharmaceutical company manufacturing sterile active pharmaceutical ingredients (API) products with a long history of over three decades of commercial manufacturing.
We are one of India’s largest producers of sterile API with an annual capacity of over 350 MT/ annum. We are the first company in the country to produce Ampicillin Sodium Sterile and other Proton Pump Inhibitors i.e., omeprazole sodium sterile and Pantoprazole sodium sterile, Esomeprazole Sodium Sterile. Our manufacturing facility is approved by ANVISA for anti-ulcer drugs and having WHO GMP & Mexican Approval. We have strong domestic presence, and we export our products to over 15 countries. We have developed a robust product range of over 20 products which include Sterile bulk API and general formulations (Vials)
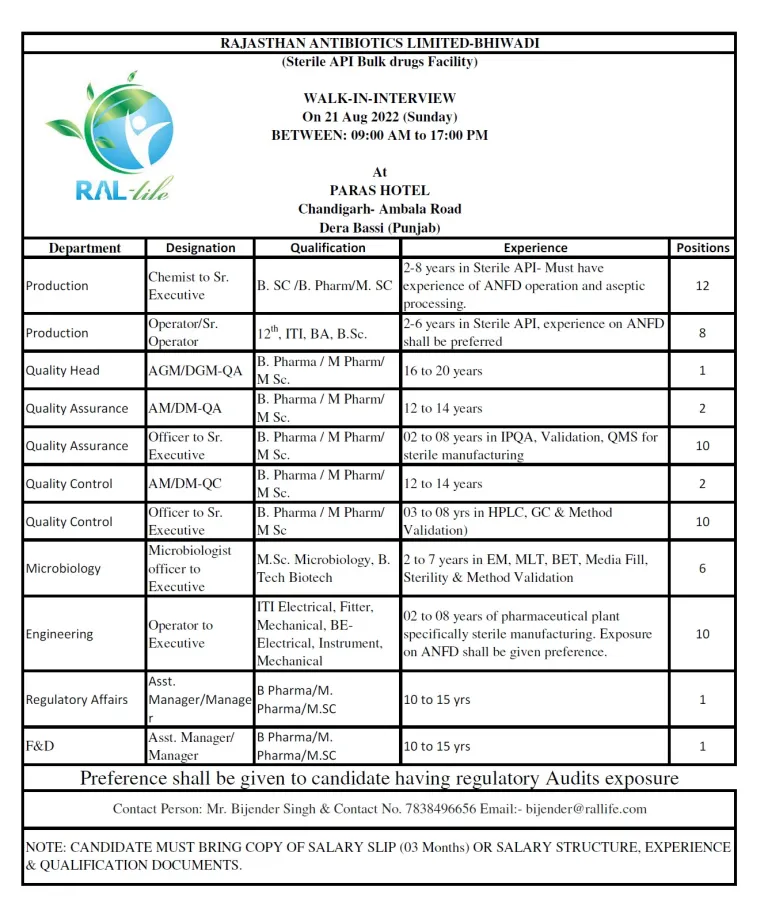
Vacancy Details
Dept. Production: – Chemist to Executive
Positions -12
Qualifications: B. SC /B. Pharm/M. SC
Experience: 2-8 years in Sterile API- Aseptic Techniques, Review of BPR/BMR documents & validation.
Dept: Engineering:- Operator to Executive (Position-10)
Qualification:- ITI Electrical, Mechanical, Fitter, Diploma, BE in Electrical, Mechanical & Instruments
Exp: 2 to 8 years of Pharmaceutical plant specifically sterile manufacturing. Exposure on AFND shall be given preference.
Dept. Regulatory Affairs: – Asst. Manager/ Manager (01 Positions)
Qualifications: B. Pharma / M Pharm/ M Sc.
Experience: 10 to 15 Years in regulatory affairs and having good knowledge of filing.
Dept. F&D: – Asst. Manager/ Manager (01 Positions)
Qualifications: M Sc. B. Pharma / M Pharm/BE Chemical
Experience: 10 to 15 Years
Dept. QA: – AM/DM (2 Positions)
Qualifications: B. Pharma / M Pharm/ M Sc.
Experience: 12 to 15 years
Dept. QA: – Officer to Sr. Executive (10 Positions)
Qualifications: B. Pharma / M Pharm/ M Sc.
Experience: 02 to 08 years in IPQA, Validation, QMS
Dept. QC: – Officer to Executive (10 positions)
Qualification: – M Sc. B Pharma, M. Pharma.
Experience: 03 to 08 yrs in HPLC, GC & Method Validation)
Dept. Micro: – Microbiologist officer to Executive (10 positions)
Qualification: – Microbiologist officer to Executive
Experience: 2 to 8 years in EM, MLT, BET, Media Fill, Sterility & Method Validation
Dept. Production: – Operator/Sr. Operator – Positions -08
Qualifications: 12th, ITI, Diploma, BA, B.Sc.
Experience: 2-6 years in Sterile API- Aseptic Techniques, Solution & Review of BPR/BMR
Dept. QA: – AGM/DGM (1 Positions)
Qualifications: B. Pharma / M Pharm/ M Sc.
Experience: 18 to 20 years
WALK-IN-Date: 21 Aug 2022 (Sunday)
Time: 09:00 AM to 17:00 PM
Venue: Paras Hotel, Chandigarh-Ambala Road, Dera Bassi(Punjab)
Contact Person: Mr. Bijender Singh & Contact No. 7838496656
Email: bijender@rallife.com