Ichor is a specialty plasma derived biopharmaceuticals company committed to innovate, develop and commercialize high-quality plasma derivatives to save lives and enhance the quality of life for the people. Ichor operates through an integrated platform of safe and reliable collection of high-quality human plasma, a state-of-the-art fractionation facility capable of producing life-saving therapies in conformance with the most stringent international standards that is supported by a cross functional team of scientists.
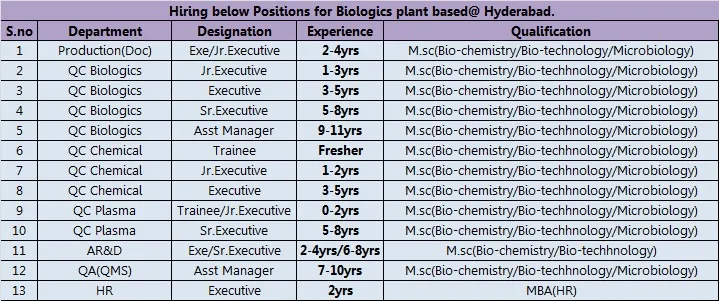
Vacancy Details
JD for Executive QC:
- Qualification: Masters in Biotechnology or Biochemistry.
- Experience: 3-4 years in Bio-Pharma Quality Control especially in Biologics QC.
1) Able to handle one specific area of work independently i.e. Instrumentation/Biochemical/Assay/electrophoresis etc. (hands on experience).
2) Should have thorough knowledge in the specialized work area (whatever work area he handles).
3) Require sound knowledge in trouble shooting with good analytical aptitude.
4) Should be very good at handling QC instruments with 21CFR complaint software’s.
5) Should have good knowledge in GXP practices.
6) Must have an overview on QMS elements and able to follow.
7) Should have an idea in usage of LIMS or any other laboratory data management software’s.
8) Able to work in shifts.
Department: Production
- Designation: Shift In-charge / Chromatography Officer / TFF / Buffer / General Documentation Officer / Trainee / Jr. & Sr. Executive
Note: For HR position we need only Female Candidates should have experience in Recruitment & Excel Knowledge.
Send your resumes to: bvrcareers@gmail.com.Shortlisted candidates will get a call from HR Dept.
Contact: 9121514444 |7337227575 |8185999958.
For More Job Updates Join Whatsapp Groups Below
Jobs zone -1 click Here
Jobs zone-2 Click Here
Jobs zone-3 Click Here
Jobs zone-4 Click Here
Jobs zone-5 Click Here

